Copper Isotopes: Properties, Applications, and Industrial Uses
Copper isotopes play a crucial role in various industrial, medical, and scientific applications. Copper (Cu) is a naturally occurring element composed of different isotopes, with the two most stable and naturally abundant being 63Cu and 65Cu. These isotopes have unique properties that make them valuable in several fields, from nuclear energy to advanced medical treatments. This article explores the characteristics, applications, and benefits of copper isotopes, highlighting their significance in modern technology and industry.
What Are Copper Isotopes?
Copper isotopes are variants of the element copper that differ in the number of neutrons they contain. The two stable isotopes of copper are:
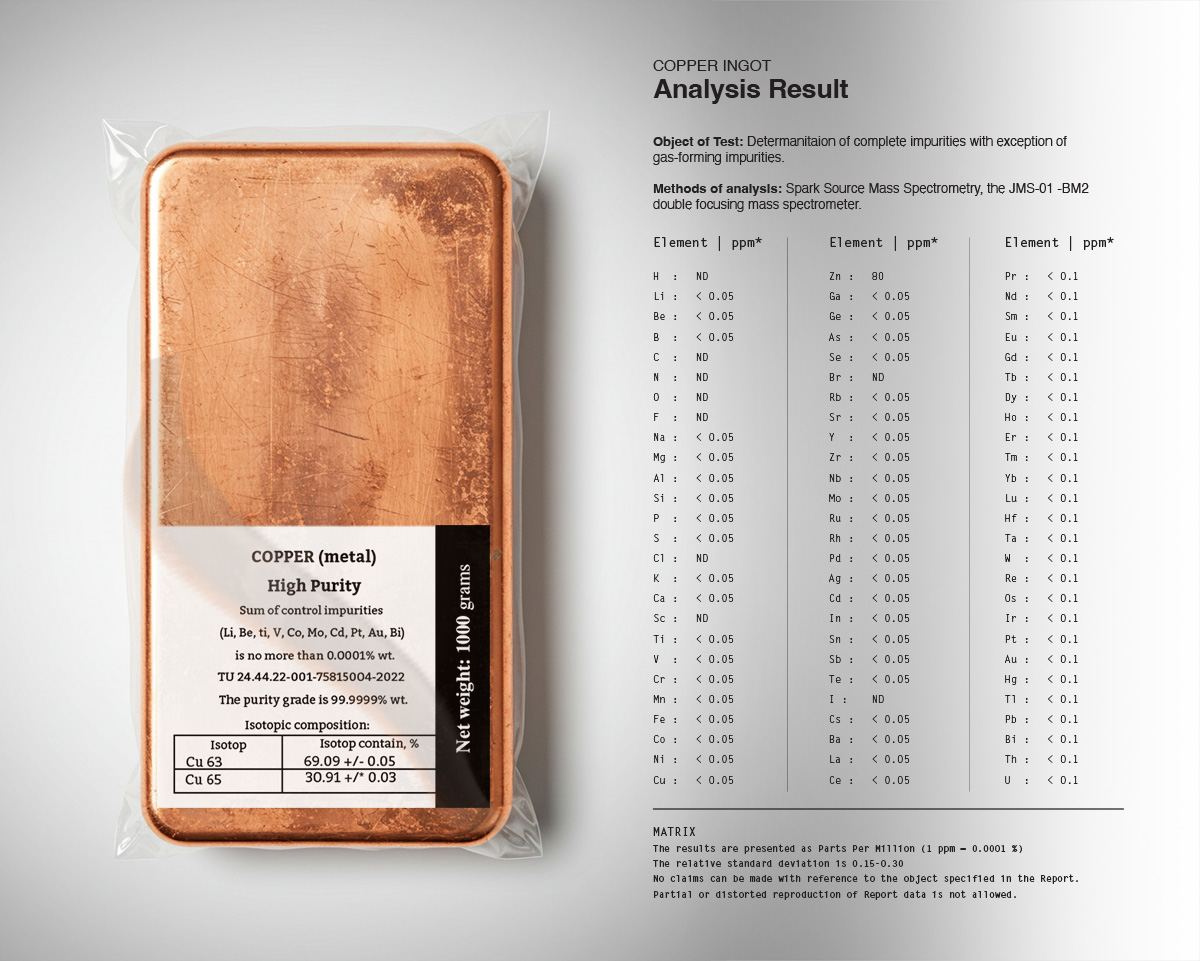
- 63Cu (69.17% natural abundance)
- 65Cu (30.83% natural abundance)
These isotopes exhibit slightly different physical and chemical properties, which can be leveraged for specialized applications. Due to their high purity and isotopic specificity, copper isotopes are widely used in industries where precise material composition is critical.
Key Properties of Copper Isotopes
- High Purity Levels: Copper isotopes are manufactured with exceptional purity to ensure precise isotopic compositions for industrial and scientific applications.
- Stable and Non-Radioactive: Both 63Cu and 65Cu are stable isotopes, meaning they do not undergo radioactive decay, making them safe for various applications.
- Superior Conductivity: Copper isotopes maintain the excellent electrical and thermal conductivity properties of copper, making them ideal for electronic and power applications.
- Chemical Stability: These isotopes exhibit high chemical stability, allowing them to be used in harsh environments such as nuclear reactors and space applications.
Applications of Copper Isotopes
Copper isotopes are utilized in multiple sectors, ranging from healthcare to high-tech industries. Below are some of the most important applications:
1. Medical and Pharmaceutical Applications
- Radiopharmaceuticals: Copper isotopes, particularly 64Cu, are used in PET (Positron Emission Tomography) imaging and cancer diagnostics. 64Cu can bind with biomolecules to target tumors, helping in early detection and treatment planning.
- Targeted Radiotherapy: Certain copper isotopes are employed in radiotherapy for treating diseases like neuroendocrine tumors and liver cancer.
- Biomedical Research: Copper isotopes help in tracing metabolic pathways and studying copper metabolism disorders.
2. Nuclear Energy and Reactor Technology
- Nuclear Reactor Control Rods: Copper isotopes are used in neutron-absorbing materials to regulate nuclear reactions in reactors.
- Nuclear Waste Management: Specialized copper isotopes are studied for their potential in safely handling and storing nuclear waste.
3. Aerospace and Space Exploration
- Spectroscopy and Astrophysics: Copper isotopes are used in spectroscopic analysis to study celestial bodies, providing insights into stellar compositions and cosmic events.
- Satellite and Spacecraft Components: Due to their thermal and electrical properties, copper isotopes are used in space-grade electronic components and radiation shielding.
4. Electronics and Semiconductor Industry
- High-Precision Electronics: Copper isotopes are incorporated into microprocessors and high-frequency circuit boards to enhance performance and stability.
- Superconductors: Copper isotopes contribute to the development of high-temperature superconducting materials for quantum computing and magnetic resonance imaging (MRI) technologies.
5. Industrial and Scientific Research
- Material Science: Isotopically enriched copper is used to study corrosion resistance, oxidation rates, and material durability.
- Nanotechnology: Copper isotopes are used in the fabrication of nanostructured materials with enhanced mechanical and electrical properties.
Future Trends and Innovations in Copper Isotope Applications
With the advancement of technology, the demand for high-purity isotopes is increasing. Some of the emerging trends include:
- Enhanced Radiopharmaceuticals: Continued research on 64Cu and other copper isotopes aims to improve cancer diagnostics and therapy.
- Quantum Computing Advancements: The use of copper isotopes in superconducting materials is paving the way for more efficient quantum computers.
- Sustainable Energy Solutions: Copper isotopes are being explored for their potential in renewable energy storage and hydrogen fuel cells.
Copper isotopes, particularly 63Cu and 65Cu, play a vital role in various industrial, medical, and scientific applications. Their unique properties, including high purity, stability, and superior conductivity, make them essential in fields ranging from nuclear energy to advanced medical imaging. As technology continues to evolve, the applications of copper isotopes will expand, leading to new innovations and breakthroughs in science and industry.
For industries and researchers looking to leverage the benefits of copper isotopes, investing in high-purity isotopic materials can provide significant advantages in performance, accuracy, and efficiency.

Why Choose BLACKSEA TRADING COMPANY?
Quality Assurance: We ensure the highest quality standards for all our products, delivering reliability and excellence.
Competitive Pricing: Our commitment to fair and reasonable pricing ensures you get the best value for your investment.
Reliable Supply: Count on us for consistent and timely deliveries, maintaining seamless operations for your business.
Exceptional Service: Our team is dedicated to providing outstanding service, addressing your inquiries promptly and effectively.
Take this opportunity to optimize your sourcing strategy and benefit from our premium offerings. Please reach out to us at info@black-sea.com.tr to discuss your specific requirements or to place an order. We’re here to assist you in every step of the process.
We look forward to continuing our successful collaboration.